The weight loss revolution is now
IT IS NOW RECOGNIZED THAT BIOLOGY-BASED APPROACHES ARE THE KEY TO WEIGHT LOSS.
Peer reviewed research conducted at the Pennington Biomedical Research Center and published in OBESITY validated that SmartByte® users are satisfied on 23% less food than non-users. These results align with the new injected pharmaceutical compounds.

Weight loss is the fastest growing category in healthcare
More than 200,000,000 US adults are overweight or obese
The two pharma companies that own metabolic hormone based weight loss have a combined value of $1.3 Trillion
Company
Novo Nordisk
Eli Lilly
MARKET CAP MAY 2022
$232B
$270B
MARKET CAP MAY 2023
$374B
$415B
SHARE PRICE MAY 2022

$103
$295
SHARE PRICE MAY 2023
$168
$436
60%+
Growth
COMPANY
Novo Nordisk
-
Eli Lilly
-
-
Share Price
Market Cap
Share Price
Market Cap
january 1, 2021
$35
$151B
$166
$158B
March 8, 2024
$136
$607B
$780
$741B
Now there is a safer, more affordable way to use GLP-1 and GIP to get a share of the largest healthcare market in history.
Scientific Intake’s proven, FDA cleared solution fills
“The Treatment Gap” for millions of consumers
Because…
- Many don’t want to inject synthetic appetite hormones when their natural ones can be activated
- Many can’t tolerate the side effects of the pharmaceutical compounds
- Many can’t afford the exorbitant cost
- Many will eventually abandon the drugs and seek ways to maintain the weight lost
The introduction of our natural solution will generate patient interest and major media attention to build awareness quickly.


Controlling Hunger, Appetite and Satiety Is the Key to Successful Weight Management
- Only placed when eating, user controlled
- FDA Cleared, designated Non-Significant Risk
- Built in sensor to track use and progress to goal Non-Significant Risk
- One time cost, plus potential monthly revenue from digital support
- Health Savings Account (HSA) eligible
- Custom device - imitators are pre-empted by the individualized aspect of the device, IP, regulatory controls and device fabrication techniques
- Drug Free – no digestive system, pancreas or thyroid side effects
- Nothing implanted in the body
- Non-systemic-Can be used alone or in combination with other weight-loss therapies
- Quality system in place, FDA audit passed
- Indicated for individuals with a body mass index (BMI) in the range 26-36 kg/m2


Our Solution
Placed only when eating, Scientific Intake’s SmartByte® decreases the capacity of the oral cavity, causing three important things to happen:
Smaller bites and thorough chewing
Increased oral processing, taste and savoring of food
Time for the stomach to signal the brain and register satiety before overeating
SmartByte® was invented by a woman with Torus Palatinus, a benign lobulation in the palate, who credited her life-long healthy BMI to her slower eating and feeling satisfied on less food. She hypothesized that a removable space occupying device would mimic her results in others. Multiple published clinical studies have validated the safety and efficacy of her simple, safe solution.
Research conducted over the past 30 years has produced a large body of science linking rapid eating to obesity.
SmartByte® is the only FDA designated non-significant risk weight management device that works at the gateway to the body. It is non-systemic and there are no side effects.
Scientific Intake’s intraoral device slows eating, which allows time for nature’s defense system against overeating to function as intended.
For additional support, every SmartByte has an embedded, proprietary microsensor that monitors compliance. This data can be uploaded to an App that monitors usage and calculates progress to goal weight as well as communicating reminders and encouragement. The app can be enhanced with robust personalized content to generate incremental and ongoing revenue.
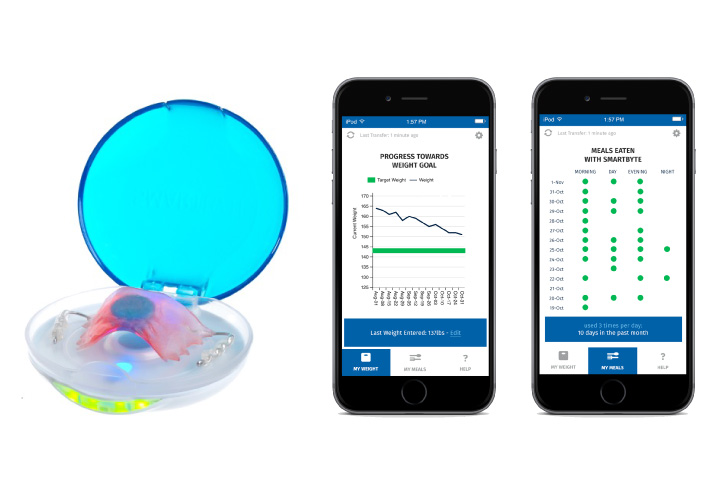

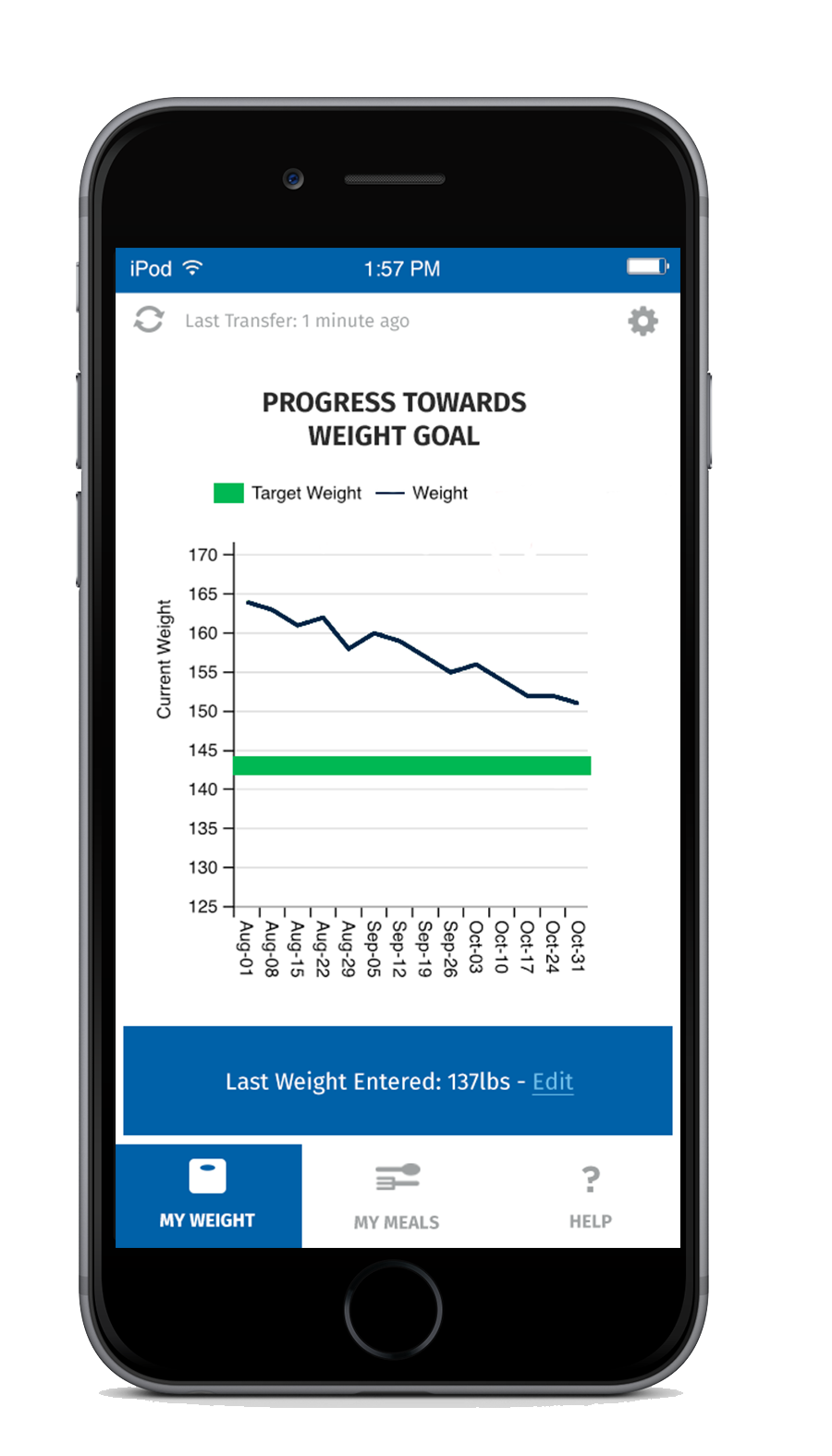
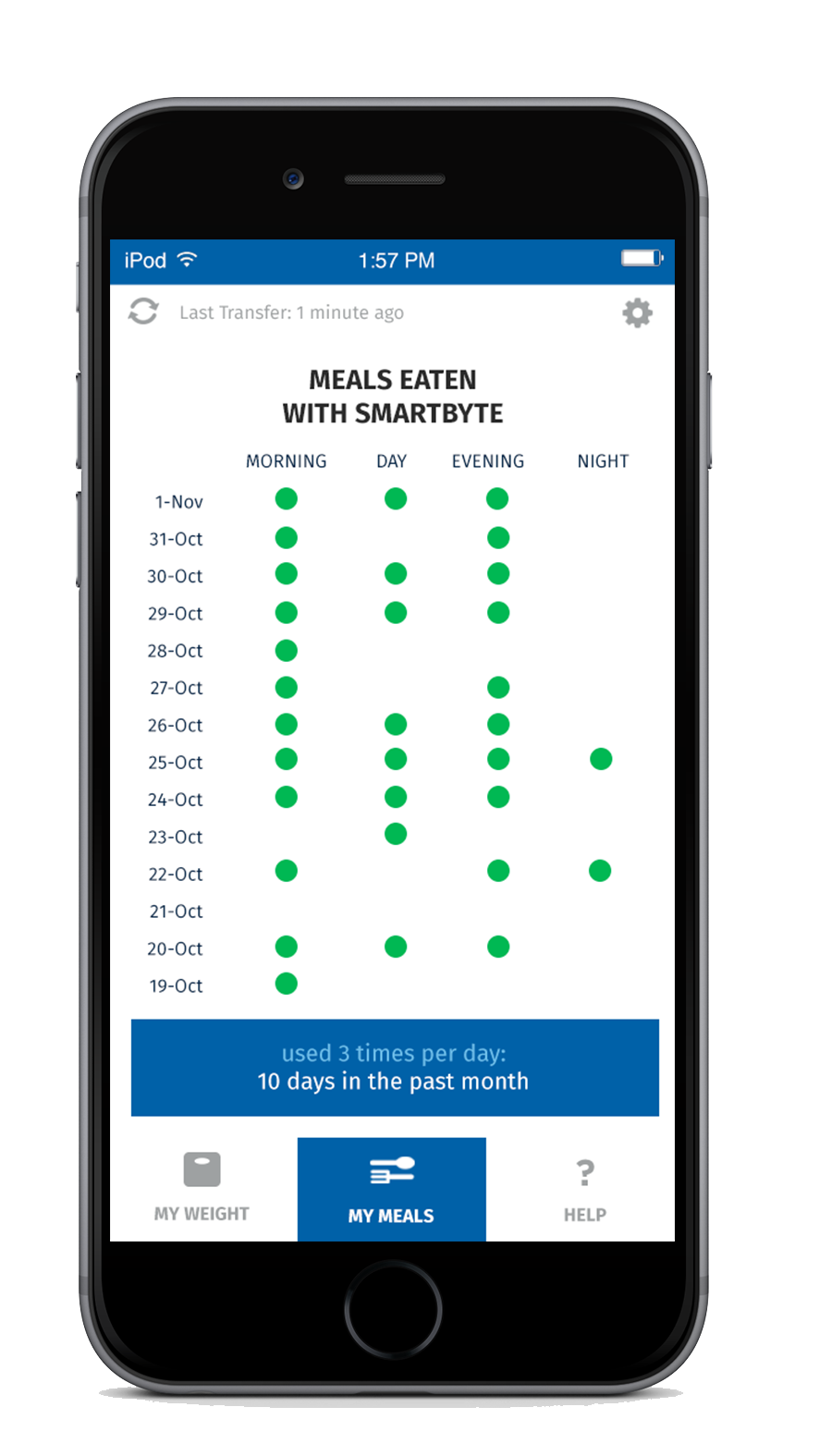
Scientific Intake’s peer reviewed, published clinical research studies and FDA clearance assure SmartByte® is safe and effective.
GLOBAL REGULATORY CLEARANCES ACHIEVED
FDA (US)
CE Mark (EU)
TGA (Australia)
Health Canada (CA)
Anvisa (Brazil)
SMARTBYTE® WILL SERVE A GREATER SOCIETAL GOOD WHILE DRIVING SIGNIFICANT PROFITABILITY
- Using a conservative user price of $1800, SmartByte® will deliver a 85% gross margin per unit
- SmartByte® is a safer, more affordable option which can appeal to a broad population. The user price of SmartByte® is less than the cost of 2 months of synthetic hormone injections
- The SmartByte® sensor and app can be further developed to drive recurring revenue or be integrated into an existing content platform
Selling just 1MM SmartByte® devices can deliver $1.8BN in gross revenue.
